Cat #: 11-725
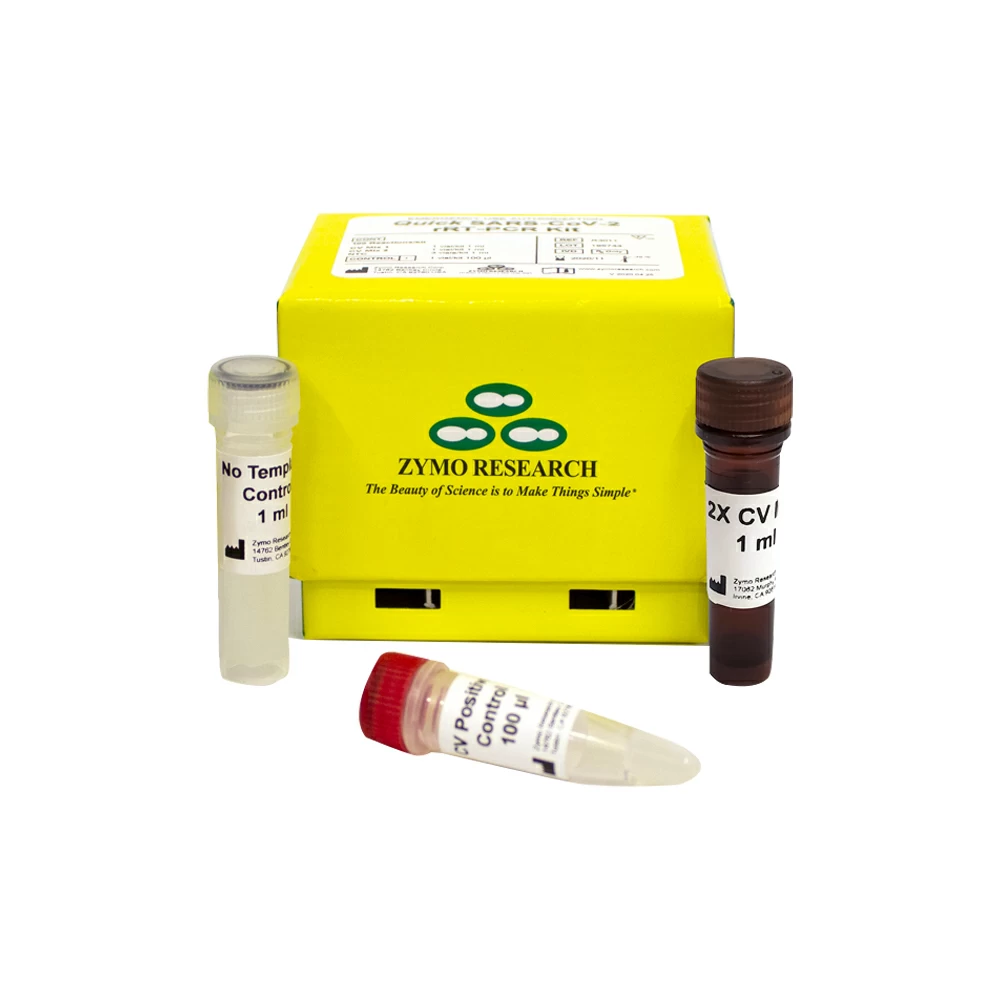
Zymo Research R3013 Quick SARS-CoV-2 Multiplex Kit (100 rxns), Zymo Research, 100 Rxns/Unit


Cat #: 11-725
Zymo Research R3013 Quick SARS-CoV-2 Multiplex Kit (100 rxns), Zymo Research, 100 Rxns/Unit
Zymo Research
100 Rxns/Unit
Brand: Zymo Research- High Sensitivity: Limit of Detection as low as 10 GEC/reaction (167 GEC/ml)
- Rapid & Easy Setup: Ready-to-use Master Mix, just add sample
- Compatible with Automated and High-Throughput workflows
$808.34
Login To Access Your Institutional Price
Zymo Research
100 Rxns/Unit
Brand: Zymo Research- High Sensitivity: Limit of Detection as low as 10 GEC/reaction (167 GEC/ml)
- Rapid & Easy Setup: Ready-to-use Master Mix, just add sample
- Compatible with Automated and High-Throughput workflows
The Quick SARS-CoV-2 Multiplex Kit is a real-time reverse transcription PCR (rRT-PCR) test for the qualitative detection of RNA from the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which is responsible for the coronavirus disease (COVID-19).
| Brand | Zymo Research Corporation |
|---|---|
| Compatibility | Compatible with automated and high-throughput workflows. |
| Equipment Required | Real-Time PCR Instruments capable of detecting HEX/VIC and Quasar® 670/Cy5 fluorophores. Adjustments of the RT-PCR parameters may be necessary for instruments different than the CFX96 Touch from Bio-Rad. |
| Input Quality | Purified RNA free of enzymatic inhibitors. |
| Processing Time | </= 2 hours from set up to results. |
| Reagents | Complete and ready to use master mix. |
| Registration Status | CE-IVD Marked |
| Sample Input Material | Purified RNA from upper respiratory and lower respiratory systems. |
This test has not been FDA cleared or approved. In the USA this test can be adopted in clinical diagnostics laboratories as an LDT according to local state guidelines. The test can be used for RUO/Informational purposes. The test is currently pending for CE IVD certification.
This test detects specifically SARS-CoV-2 and cannot be used to detect other viruses or pathogens.
Please refer to the Interpretation of Results, the Appendix, and the Troubleshooting Guide sections in our Quick SARS-CoV-2 Multiplex Kit protocol.
Sometimes aberrant qPCR signal are observed. Please refer to the Interpretation of Results, the Appendix, and the Troubleshooting Guide sections in our Quick SARS-CoV-2 Multiplex Kit protocol.
This may indicate incorrect plate set-up or a contamination in the No-Template Control or in the 2X CV Mix. We recommend repeating the test, and if the problem persists to use a new aliquot of reagents.
If using RT-PCR instruments different than the CFX96 Touch from Bio-Rad, this problem can be solved by adjusting the RT-PCR parameters (e.g. baseline threshold, temperature ramp, ...). This problem may also indicate incorrect plate set up of the compromise of Quick SARS-CoV-2 reagents.
Enjoy our products? Leave a review and let us know.


 Q1: Is the Quick SARS-CoV-2 Multiplex Kit a diagnostic test?
Q1: Is the Quick SARS-CoV-2 Multiplex Kit a diagnostic test?