Cat #: 11-654
Zymo Research R3011-10K Quick SARS-CoV-2 rRT-PCR Kit, Bulk (100ml) Component Reagents Format, 10,000 Tests/Unit
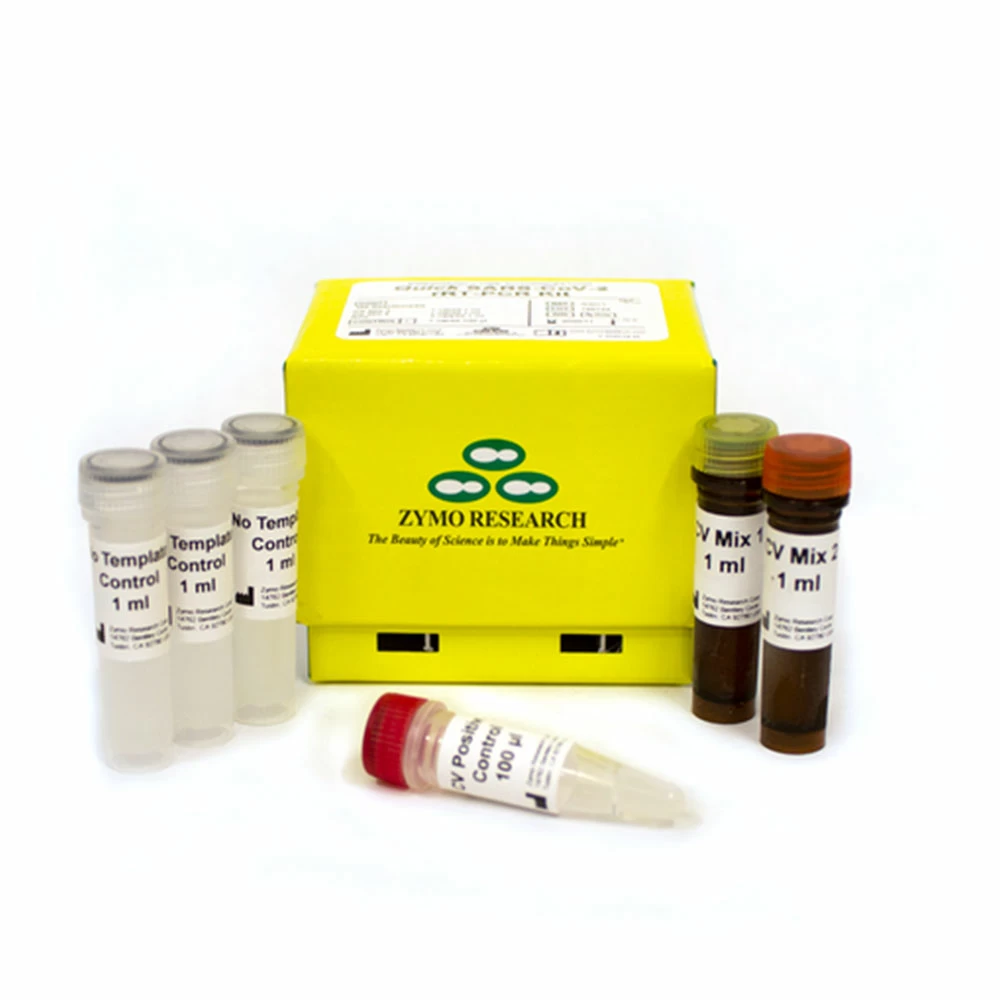


Cat #: 11-654
Zymo Research R3011-10K Quick SARS-CoV-2 rRT-PCR Kit, Bulk (100ml) Component Reagents Format, 10,000 Tests/Unit
Bulk (100ml) Component Reagents Format
10,000 Tests/Unit
Brand: Zymo Research- High Sensitivity: Limit of Detection as low as 15 copies/reaction
- Rapid & Easy Setup: Ready-to-use Master Mix, just add sample.
- Compatible with Automated and High-Throughput workflows.
- Equipment Required: Real-Time PCR Instruments capable of detecting HEX™ and Quasar® 670/Cy5 fluorophores.
- Input Quality: Purified RNA free of enzymatic inhibitors.
- Processing Time: less than or 1.5 hours from set up to results.
- Reagents: Complete and ready to use master mixes provided in 100ml bottles - Reagents are stable for up to five (5) freeze-thaw cycles.
- Sample Input Material: Purified RNA samples isolated from upper respiratory and lower respiratory systems.
$189,213.04
Login To Access Your Institutional Price
Bulk (100ml) Component Reagents Format
10,000 Tests/Unit
Brand: Zymo Research- High Sensitivity: Limit of Detection as low as 15 copies/reaction
- Rapid & Easy Setup: Ready-to-use Master Mix, just add sample.
- Compatible with Automated and High-Throughput workflows.
- Equipment Required: Real-Time PCR Instruments capable of detecting HEX™ and Quasar® 670/Cy5 fluorophores.
- Input Quality: Purified RNA free of enzymatic inhibitors.
- Processing Time: less than or 1.5 hours from set up to results.
- Reagents: Complete and ready to use master mixes provided in 100ml bottles - Reagents are stable for up to five (5) freeze-thaw cycles.
- Sample Input Material: Purified RNA samples isolated from upper respiratory and lower respiratory systems.
This product is regulated and only available to authorized clinical and diagnostic laboratories when sold for Zymo’s Emergency Use Authorization (EUA) of May 07, 2020. However, sales for research use purposes outside the EUA or to clinical and diagnostic laboratories for validated alternate workflows may be permitted. PLEASE READ THE ""INSTRUCTIONS FOR USE"" ON ""USEFUL DOCUMENTS"" BELOW PRIOR TO PURCHASING. SUBMISSION OF AN ELECTRONIC INTENDED USE FORM WILL BE REQUIRED PRIOR TO SHIPMENT. The Quick SARS-CoV-2 rRT-PCR Kit is a real-time reverse transcription PCR (rRT-PCR) test for the qualitative detection of RNA from the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which is responsible for the coronavirus disease (COVID-19). The kit targets three different regions of the viral nucleocapsid (N) gene and a host-specific target region (human RNase P gene) to assess sample quality. It includes CV Positive Control that enables assay performance monitoring, and a No-Template Control to confirm the absence of contamination in the reagents. The Quick SARS-CoV-2 rRT-PCR Kit can be used on purified RNA samples isolated from upper respiratory and lower respiratory systems. It offers high sensitivity, with a limit of detection as low as 15 viral genome equivalent copies per reaction, a fast turnaround time of less than 1.5 hours and a simple workflow in which RNA is simply added to the kit reagents and directly analyzed. Due to its simplicity, set-up up can be performed in automation and the kit is compatible with high-throughput platforms. The kit is compatible with multiple Real-Time PCR instruments and technical support is available for all steps of the setup process and data interpretation.
This test has not been FDA cleared or approved; This test has been authorized by FDA under an EUA for use by authorized laboratories; This test has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens; This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb- 3(b)(1), unless the authorization is terminated or revoked sooner. Qualitative assay for use on the Bio-Rad CFX96 Real-Time PCR Detection System. For use under the FDA Emergency Use Authorization (EUA) only. For in vitro diagnostic use.
| EUA Letter | https://www.fda.gov/media/137781/download |
|---|---|
| Instructions for Use | https://geneseesci.com/wp-content/uploads/2020/06/Instructions-for-Use-Quick-SARS-CoV-2.pdf |
| King Fisher Script | https://geneseesci.com/wp-content/uploads/2020/06/sars_cov-2-rna-king_fischer_script.pdf |
| Intended Use Form | https://zymoresearch.wufoo.com/forms/r3011-intented-use-form/ |
Enjoy our products? Leave a review and let us know.

