BIOFIL™ FFP2 Particle Filtering Mask
Guangzhou BIOFIL Air Purification Materials Co.,Lt mask model MY3D2 is an FDA authorized imported respirator, verifiable on the FDA website Appendix A list. BIOFIL™ disposable protective masks meet or exceed the requirements of EN 149:2001 A1:2009 European standard for FFP2 masks and comply with the requirements of EU regulation (EU) 2016/425 for Personal Protective Equipment. Regulation (EU) 2016/425 EU type-examination certificate and EN 149 test report are available upon request.
• Standard: EN149:2001 A1:2009, FFP2
• Non-woven fabrics and melt blown filter layer
• Earloops constructed of spandex and nylon
• Manufactured in ISO 13485 compliant facility
 |
FEATURES
| ||||||
Questions or Need Any Help?
Call us today at 800.789.5550!
Disposable Face Masks
According to FDA Emergency Use Authorization (EUA), non-surgical facemasks are authorized under the EUA when they are intended for use by members of the general public, including HCP in healthcare settings as PPE, to cover their noses and mouths, in accordance with CDC recommendations, to prevent the spread of the SARS-CoV-2 during the COVID-19 pandemic.
• Blocks exhalation or ejection of pollutants from the oral and nasal cavity
• Single-use disposable face masks, 6.88in x 3.74in (175mm x 95mm)
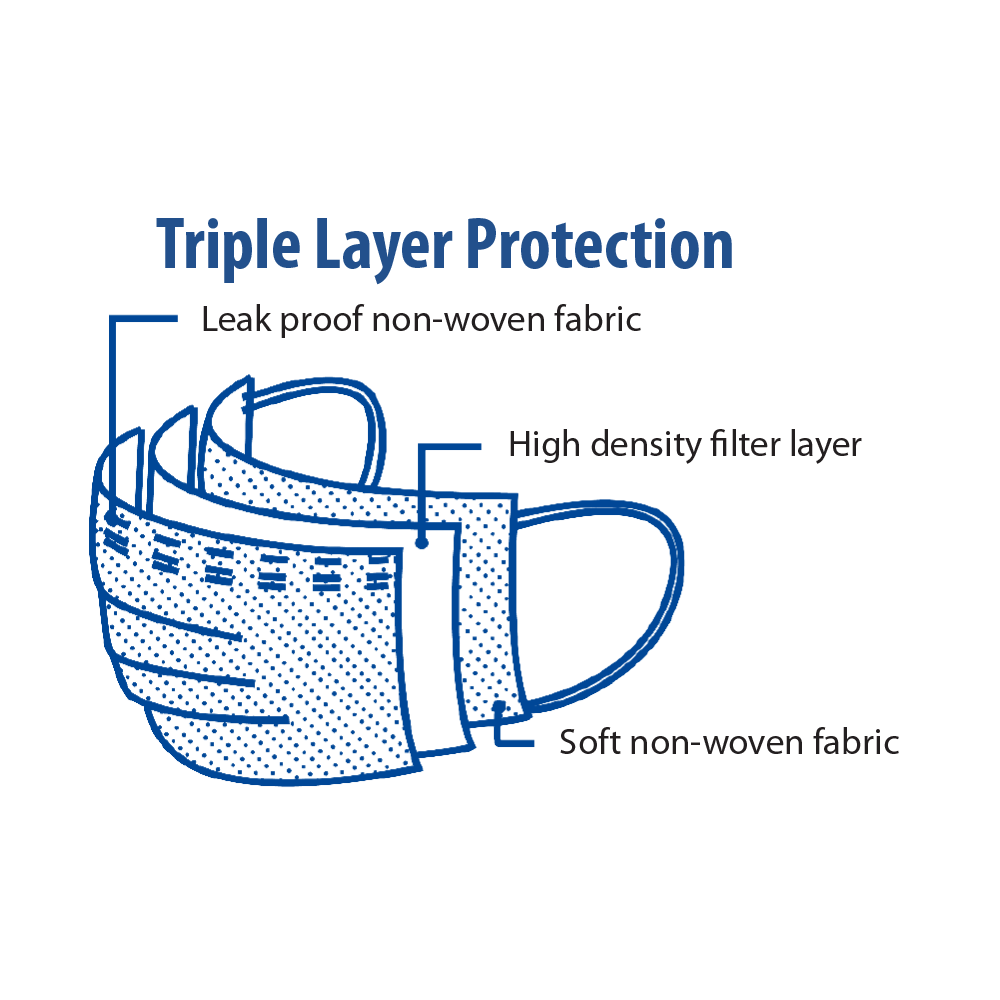
• Soft, non-woven fabric, high density filter layer, and nose clip
 |
 |








